远红外伤痛贴(临床款)
远红外伤痛贴(临床款)
远红外伤痛贴(临床款)
- 产品详情
- 产品参数

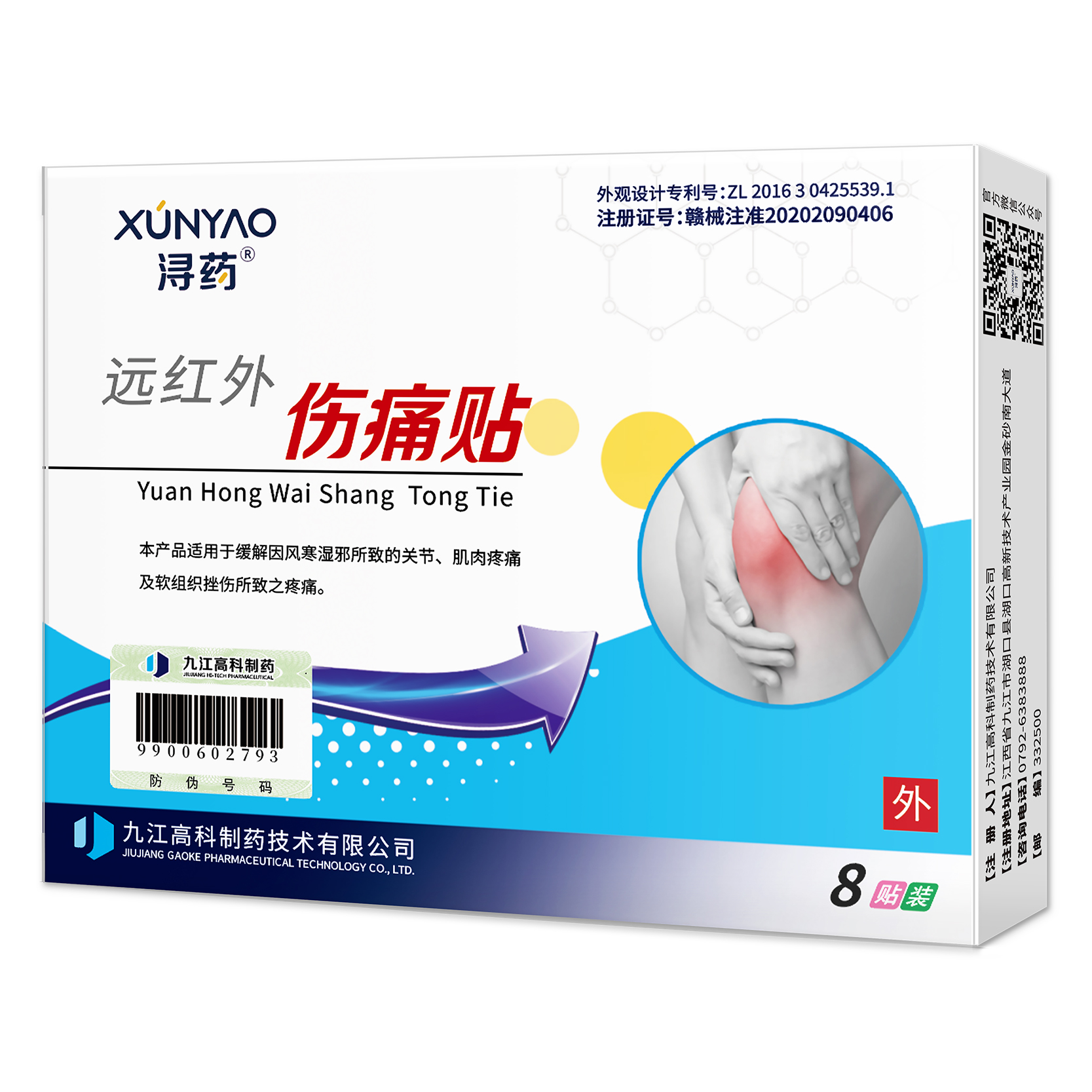
【产品名称】
通用名称:远红外伤痛贴
汉语拼音:yuanhongwaishangtongtie
【规格型号】
80mm×105mm
【结构及组成】
产品由基衬、珠光膜、无纺布、基质、离型纸组成。基衬由医用胶布(平纹无纺布涂胶材料)、基质由(远红外粉、医用压敏胶、磁片)组成。
【产品性能】
外观平展整洁,切边整齐,无脱胶、漏胶,无破损,无污染。医用压敏胶为棕褐色,气味芳香。胶面涂布均匀,色泽一致,背面无渗漏。
【作用机理】通过穴位贴敷,通经活络、祛湿除寒、活血止痛。如风湿性关节炎、颈椎病、肩周炎、腰肌劳损、腰腿痛、扭伤、挫伤等疼痛。
【适用范围】适用于缓解因风寒湿邪所致的关节、肌肉疼痛及软组织挫伤所致之疼痛。
【使用方法】外用。使用时保持患处皮肤洁净,将外贴揭去离型纸,贴于患处(即阿是穴),每贴使用6-8小时或遵医嘱。

【禁忌症、注意事项】
1、孕妇、高度体质过敏者、高热患者、糖尿病患者禁用;
2、代偿不全的心脏病患者以及使用心脏病起搏器者禁用;
3、皮肤破损者、有出血倾向或损伤后出血不止者禁用;
4、急性损伤请在二十四小时后使用;
5、若出现红疹或皮肤过敏,请缩短使用时间或暂停使用;
6、儿童须在成人监护下使用。
【贮 藏】室温下密封保存
【生产日期】见产品标签
【有效期】两年
【注册证号】赣械注准20202090406
【产品技术要求】赣械注准20202090406
【生产许可证号】赣药监械生产许20150073号
【生产企业】九江高科制药技术有限公司
【注册人】九江高科制药技术有限公司
【注册地址】江西省九江市湖口县湖口高新技术产业园金砂南大道
【生产地址】江西省九江市湖口县湖口高新技术产业园金砂南大道
【咨询电话】0792-6383888
【邮 编】332500
【注册人联系方式】0792-6383999
【全国营销中心地址】广州市海珠区广州大道南敦和路海珠科技创业园189号D栋3楼南座
【咨询电话】020-84216188 84216189
【邮 编】510230
----------------------------------------------------------------------英文说明书------------------------------------------------------------------
Far-infrared plaster forpain
(acupoint application)
[ProductName] Far-infraredplaster for pain
[Chinesephonetic alphabet] yuan hong wai shang tong tie
[Specification] 80mm*105mm
[Composition] Theproduct contains base material, pearlized film, nonwovenfabrics and release paper. Base material consists of far-infrared powder andmedical heat-melting adhesive.
[Description] Appearanceof the product is flat and the cutting edge is neat,nondegumming, nonleak adhesive, nondestructive and nonpollution. Medical heatmeltingadhesive is tan and aromatic. Surface of adhesive is welldistributed, colourand lustre is consistent, and no leakage on the back.
[Mechanism] The product is effectivefor expediting channel, activating meridian, activating blood and relievingpain through applying on acupoints.
[Application]Theproduct is applicable for alleviating the pain associated withrheumatoid arthritis, cervical syndrome, shoulder periarthritis, lumbar musclestrain, lumbar and leg pain, sprain and contusion.
[Directions] The product is for external use. Keep affected area clean whenuse, remove release papers,stick it onto affected area (Ashi acupoint).
Each plaster can be used for 6-8 hours or consult yourdoctor.
[Warnings& Cautions]
1.Pregnant & lactation women,highly allergicperson,patients with hyperthermia, and diabetics are forbidden
2. It cannot apply for patients who suffer from heartdisease
3.Patients with skin breakage, bleeding tendency orbleeding after injury are prohibited.
4. Acute injury should be used after 24 hours
5. Shorten using time or stop using if skin rash orallergy.
6.Children should be used under adult supervision.
[Storage] Storein sealed conditionunder room temperature.
[Package] 2 plasters per box
[Manufactringdate] Seeproduct label
[Validity] 24 months
[Registrationnumber] GXZZ 20202090406
[Producttechnical requirements] GXZZ 20202090406
[Productionlicense No.] GSYJXSCX 20150073
[Producer] JiuJiang GaokePharmaceutical Technology CO., LTD
[Registrant] JiuJiang GaokePharmaceutical Technology CO., LTD
[Producer]
[Productionaddress] Jinsha South Avenue,Hukou high tech Industrial Park, Hukou County, Jiujiang City, Jiangxi Province, China
[Consultingtelephone] 0792-6383888
[Post code] 332500
[Web site] www.jjgaoke.com
[Contactinformation of Registrant] 0792-6383999
[Compile time] December20th, 2014